Left lateral sectionectomy
Gregory Sergeant
Introduction
Although laparoscopic liver surgery is around the block for more than 20 years, many HPB-trained surgeons are still reluctant to its introduction in their practice. The first laparoscopic left lateral sectionectomy (LLS) was performed in 1996 (1).
In spite of the early criticism, one of the conclusions of the first international consensus conference on laparoscopic surgery was that the laparoscopic approach for LLS should be considered the ‘gold standard’ (2).
Indeed, LLS is an uncomplicated anatomical liver resection with little or no morbidity that maximizes the benefits of the laparoscopic approach. After 2008, the ‘laparoscopic’ HPB surgical community was convinced that laparoscopic LLS should be offered to all patients needing to undergo resection of segment 2 and 3. The result was that the well-anticipated prospectively randomized controlled Orange II trial was stopped prematurely owing to slow patient accrual for the study (3). Nevertheless, a recent population-based study from France showed that between 2007 and 2012 only 28.5% of LLS procedures were performed laparoscopically leaving room for wider adoption in the future (4).
Anatomy
Several anatomical landmarks are encountered during a LLS. These landmarks may be visualized on the liver surface or during parenchymal transection. The transection plane is usually on the left of the falciform ligament as this surface landmark divides segment 4 from the left lateral sector. In my experience especially the anatomy of the portal pedicle to segment 3 (P3) may vary in its branching pattern, either as a separate pedicle, either a separate subsegmental branches coming of the left main portal pedicle.

Operative technique in 7 steps
A Laparoscopic LLS may be broken down into 7 steps.
The first step consists of exposing the left lateral segments, lysis of any adhesions, mobilisation if deemed necessary and intraoperative ultrasound.
The second step consists of isolating the portal pedicles to segment 2 and 3.
The third step consists of division of portal pedicles after clipping with scissors or stapling.
The fourth step consists of transecting the remaining hepatic parenchyma.
In a fifth step the left hepatic vein is divided with a linear stapler.
In the two final steps perfect hemostasis and bilostasis is obtained and the surgical specimen is extracted via an incision away from the subcostal margin
Step 1. Exposure
A pneumoperitoneum of 12-15 millimeters Mercury (mmHg) is created. Next 4 to 5 trocars are placed. I usually use an 11mm trocar on the midline and two 12mm trocars laterally in order to have 2 options to insert laparoscopic staplers.
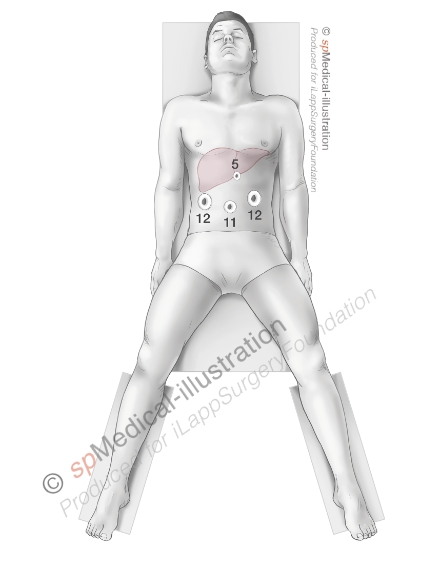
After inspection of the liver and thorough ultrasound examination you may proceed either with complete or minimal mobilisation of the left lobe depending on the exposure of the left side of the round ligament/umbilical part of the left portal vein. It may sometimes be necessary to mobilize the liver in case of attraction of the omental fat to a large tumor.
I always take down the left triangular ligament, round ligament of the liver and the falciform ligament at the start of the procedure. For me the avantage of having a more mobile left lobe outweighs the disadvantage of loosing some of the natural retraction.
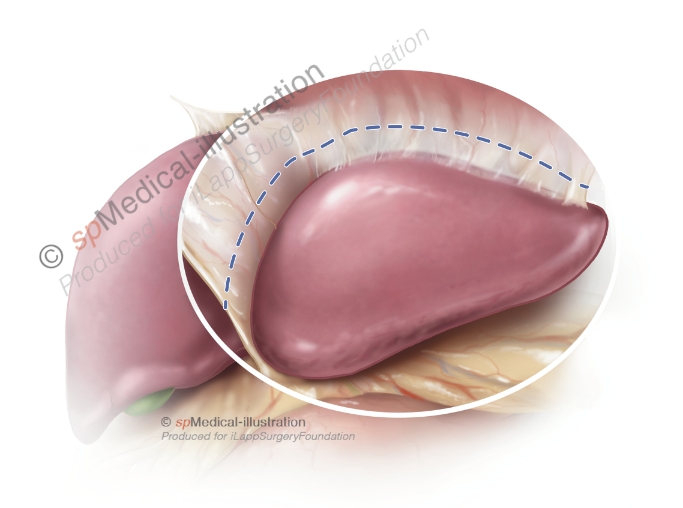
Step 2. Isolation of portal pedicles to segment 2 & 3
In the second step the portal pedicles to segment 2 and 3 are dissected. Generally, the liver parenchyma is transected ventrally and dorsally of the pedicles with ultrasonic or bipolar dissection devices. In addition the pedicles may or not be isolated by CUSA dissection in order to place clips. Other surgeons deliberately avoid dissection close to the pedicles.
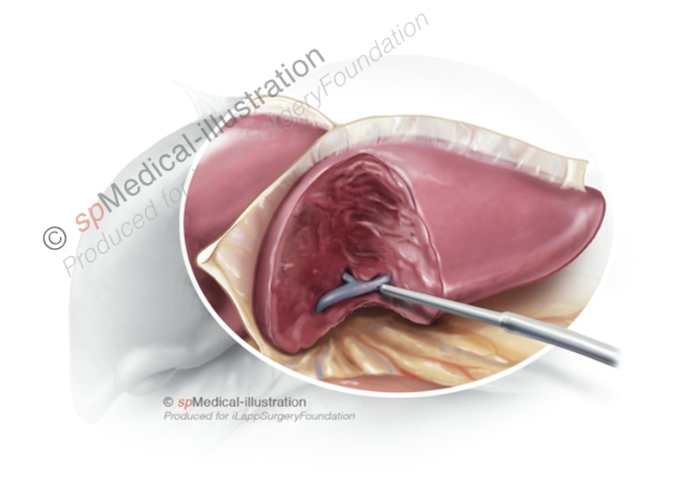
Step 3. Division of portal pedicles to segment 2 & 3
Portal pedicles may be either be clipped or stapled. When pedicles are small and well isolated with CUSA they may be clipped. I prefer using Hem-o-lok type plastic clips in different sizes depending of the size of the pedicle.
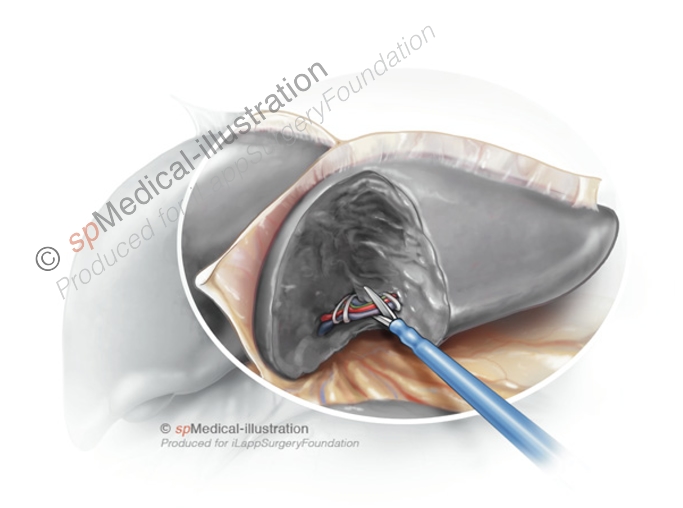
I usually double clip on the right side. Next the pedicles are cut with scissors. Alternatively, the pedicles may be stapled with endoscopic staplers with vascular cartridge. This may be impossible when the tumoral lesion is located close to the pedicle.
Step 4. Parenchymal transection
The remaining parechyma is transected in a ventral to dorsal and caudal to cranial direction along the falciform ligament. The central vein may be encountered and followed up to the left hepatic vein. Parenchymal transection may be performed using any of the available devices.
Step 5. Stapling of the left hepatic vein
At the cranio-dorsal part of the parenchymal transection care must be taken to avoid injury to the left hepatic vein. The left hepatic vein is stapled with an endoscopic 45 or 60mm vascular stapler that is inserted via the right 12mm trocar.
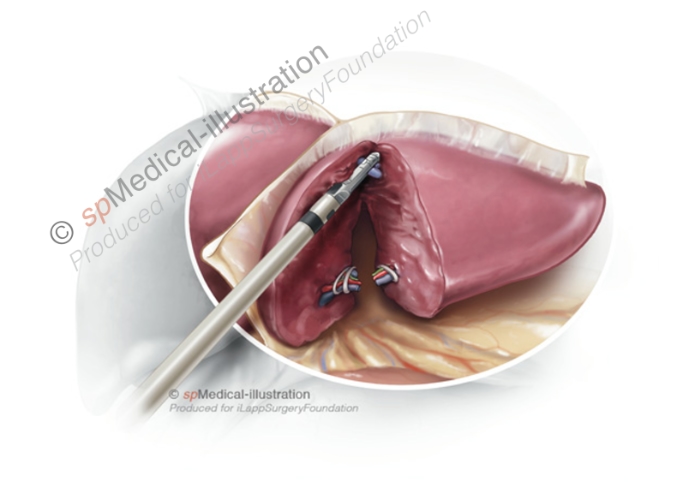
When firing the stapler, the surgeon should avoid important traction on the left lobe. I always check before firing that the stapler is well placed and the hepatic vein may be divided with a single load. A sling around the left lobe may help with the placement of the stapler by pulling the left lobe away from the diaphragm.
Step 6. Hemostasis & Bilostasis
Step 6 includes careful hemostasis with a bipolar forceps and compression with a small gauze of the cut surface of the liver. The stumps of portal pedicles are inspected for bile leaks. Finally the transection plane is covered with a hemostatic patch or glue. No drains are needed.
Step 7. Extraction of the specimen
The procedure is concluded with extraction of the surgical specimen in a retrieval bag.
The retrieval bag should be of a sufficient size and strength. Manipulating the specimen in the bag is aided when the retrieval bag is attached to a cylindrical tube to cast the specimen. We prefer to use a Pfannenstiel for extraction.
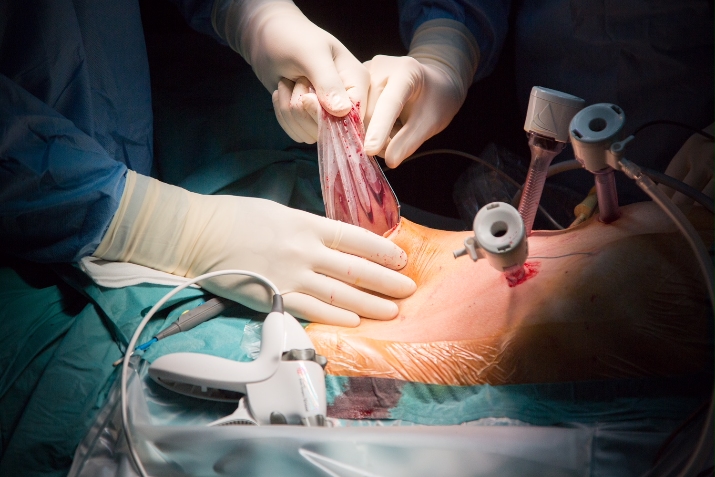
Care should be taken to avoid iatrogenic injury to the underlying bowel when opening the peritoneum. Oral diet may be reinstalled the evening of the procedure. Patients are usually discharged on postoperative day 2.
Future perspectives
In the near future in experienced teams patients undergoing LLS could benefit from ambulatory surgery management (5) and LLS for adult-to-child living donor liver transplantation could even become a new standard practice for retrieval of left lateral section liver grafts (6).