International TaTME Registry
Marta Penna
Roel Hompes
Innovation and research allow surgical techniques and patient care to advance with the relentless strive to achieve better outcomes for our patients. However, the safety profile of any new technique must be assessed carefully and its outcomes monitored closely. The International TaTME Registry was established in July 2014 in the United Kingdom and funded by the Pelican Cancer Foundation. It is accessed via the Low Rectal Cancer (LOREC) website, providing a secure encrypted online platform. The aims of the registry are, firstly, to “do no harm” to our patients through a safe, responsible and monitored introduction of this new technique. It also provides the largest cohort of TaTME cases performed worldwide, that allows regular data analysis and promotes collaboration and sharing of experiences amongst surgeons. The registry does not replace randomised controlled trials but rather provides useful information and current outcomes from the “real world” colorectal community performing TaTME that can help with the planning of the study design for trials.
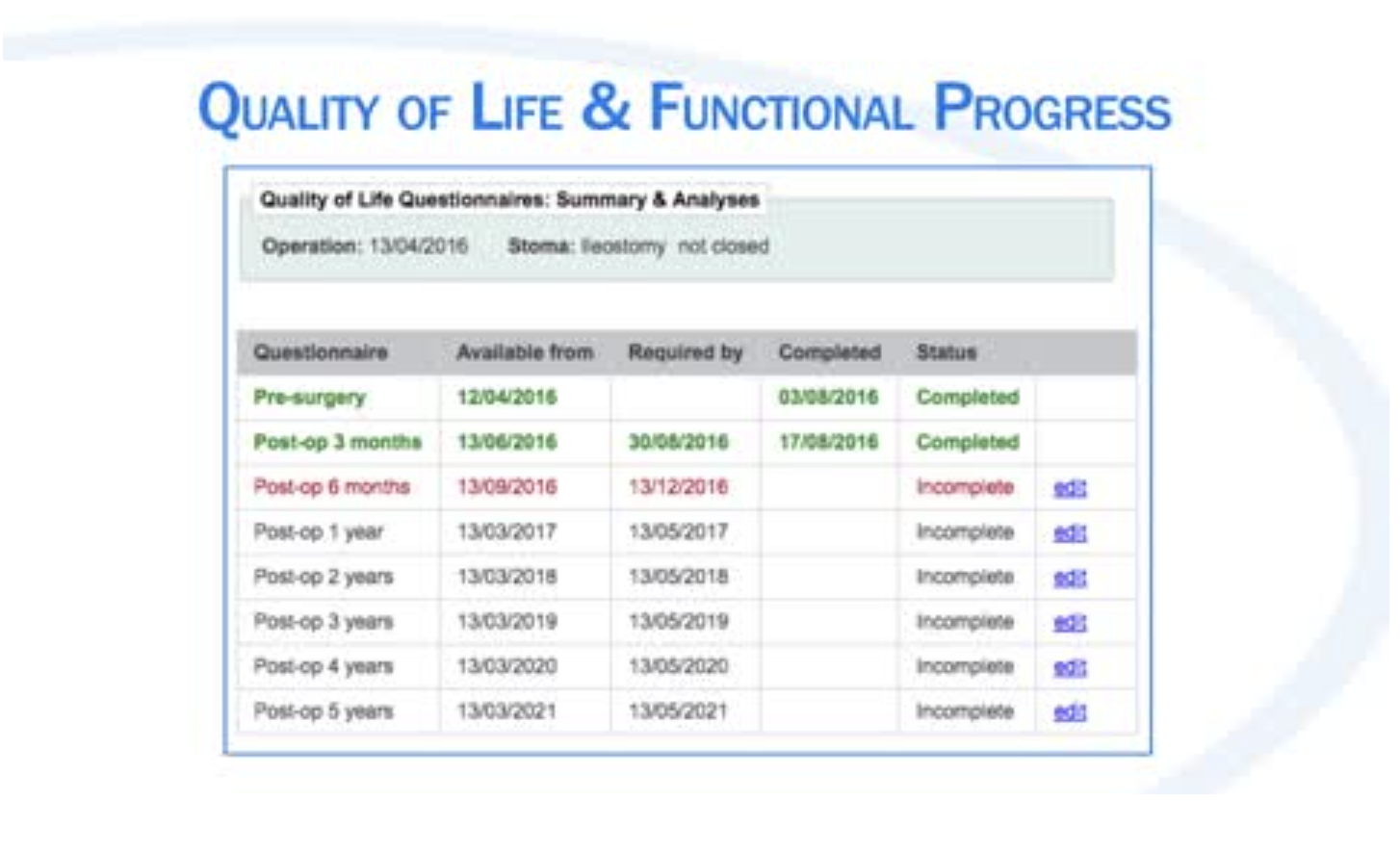
Data is recorded by navigating through 10 sections that cover various aspects of the patient’s care pathway. The database is easy to use with dropdown menus and a list of definitions explaining important variables. More recently added features include the possibility of uploading images of the TME specimen as well as the Quality of Life (QoL) and functional outcomes module. This was made possible by generous donations and translations into different languages from collaborating centres listed on the registry. The database will now, not only be able to capture the surgical practice and short-term results, but also the very important long-term oncological and functional outcomes following TaTME. This rich source of data has so far produced two publications including the first 720 recorded cases and a paper focusing on anastomotic-related morbidity. Many more aspects and topics are planned, including a learning curve analysis and 3-year oncological outcomes. All surgeons and centres that have contributed cases used in the analyses are named as part of the TaTME registry collaborators group on all published papers. Each centre can also download the data for their own hospital and publish or present their results as they wish.
Training and proctoring of a new technique is crucially important in order to allow a more accurate assessment of the operation’s true risks and benefits. The TaTME registry provides surgeons with the opportunity to track their progress in performing TaTME and identify steps or phases of the operation that require more thought and practice. This is accomplished by completing Global Assessment Scoring forms (GAS), whereby the trainee can either track their own progress but also compare their scores to those assigned by the mentor, if proctored during the case. Whether a case is proctored can also be recorded on the registry. A dedicated international TaTME educational collaborative website has been launched and can be found on www.tatme.surgery, providing useful educational resources for both healthcare professionals and patients as well as access to the registry and information on the COLOR III trial.
Finally, if you would like further information about the TaTME registry or are keen to join, please email Dr Roel Hompes (roelhompes@gmail.com) and Miss Marta Penna (m.penna@doctors.org.uk). We look forward to a strong and valuable collaboration as we continually seek to improve patient care together.